Already Established UDI
Governance Processes?
Navigate the complexities of global regulations with ease:
UDI Connect brings your processes across the finish line to global markets like
US (GUDID), EU (EUDAMED), China (CUDID) or Australia (AusUDID) by automating validations and submissions - all with one seamless API-driven solution.
Managing Global UDI Connectivity Is Hard...
Diverse Market Requirements
Data Silos and Fragmentation
Time-Intensive
Workflows
Time-Intensive Workflows
... And Doing It On Your Own, Manually, is Even Harder
Complexity
Resource Constraints
Risk of Errors
Connect. Submit. Done. UDI Compliance Your Way
UDI Connect’s API-driven approach bridges the last mile to global regulatory databases by:
Integration
Connect with your own developed or standard systems like ERP, PLM, or RIMs.
Automation
Reduce IT workload by automating validation, submission, and monitoring.
Security and Compliance
Ensure secure data handling with audit-ready logs and regulatory alignment.
Real-Time Monitoring
Gain visibility into submission statuses and compliance with instant updates.
Scalability
Adapt to evolving regulations and new markets with dynamic schema mapping.
Industry Leaders Who Trust p36 Solutions
Let's Elevate Your Robust UDI Processes with UDI Connect.
Multiple portals, diverging requirements, and evolving regulations from different regulatory databases (like GUDID or EUDAMED) are slowing you down. And ensuring your data is accurate before submission is challenging, often causing manual work, errors, and delays.
UDI Connect bridges exactly this last mile for you by validating UDI data in real time, integrating with your systems to ensure error-free submissions, reducing manual effort, and keeping you ahead of regulatory changes.
Rapid Onboarding
Get started fass. Your team is up and running in minutes, with guidance available when you need it.
Global Compliance
Always be on top of regulatory evolvements and submit to multiple authorities.
Time Savings
Focus on your strategic priorities by reducing manual effort on repetitive tasks.
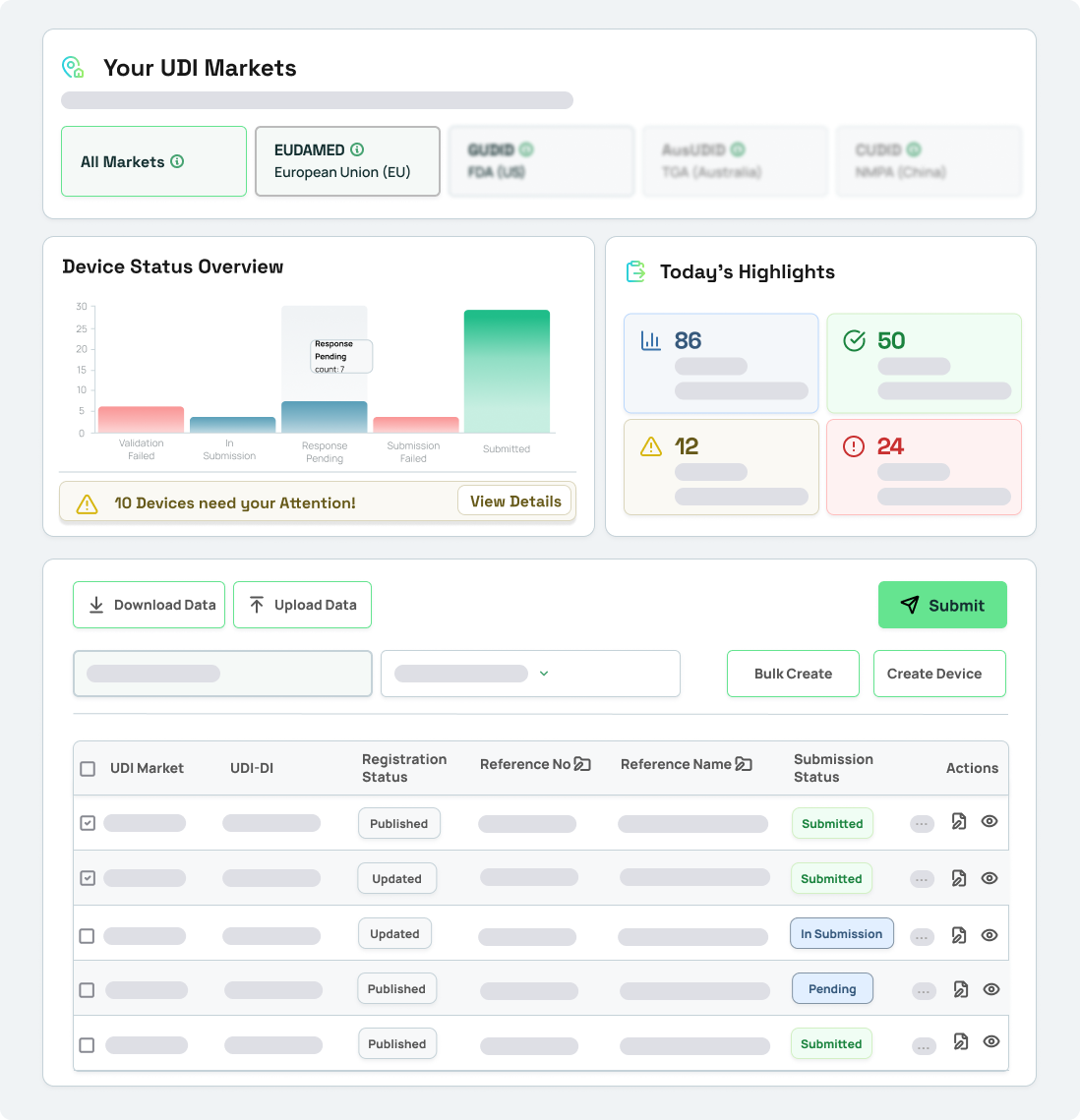
How it works
Experience a new era of global UDI compliance with UDI Connect. Our solution automates the validation and submission processes across different regulatory authorities. Say goodbye to manual errors and hello to worldwide markets.
1. Connect Instantly
Prepare your UDI data. Connect your systems easily with global regulatory database via API-interface and upload your data.
2. Validate Automatically
Uncover errors by automated validation checks against
market-specific requirements.
3. Submit Effortlessly
From EUDAMED to AusUDID: Submit directly to the required regulatory database - error-free and on time.
4. Track & Optimize Continuously
Track submission statuses in real time within your own systems. Gain insights into your progress and identify improvements.
Achieve Global UDI Compliance with UDI Connect
UDI Connect simplifies the complexities of global UDI compliance, enabling you to manage, validate, and submit UDI data seamlessly across multiple markets.
