Put UDI compliance
on autopilot.
Two solutions - one common goal:
centralize your data, automate submission, ensure global compliance effortlessly.

UDI Connect and UDI Hub
Pick the right fit for your UDI Compliance
Integrate with
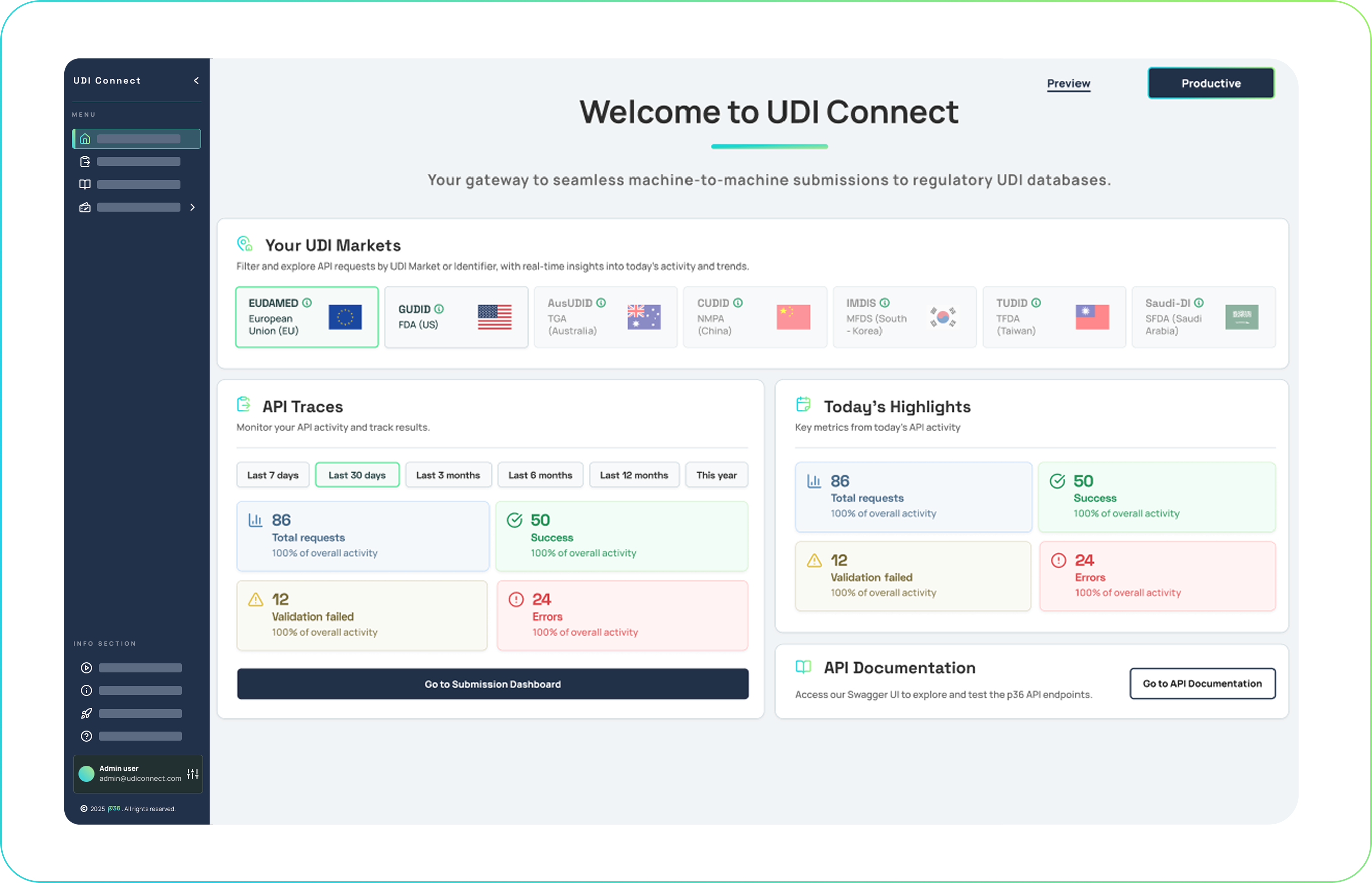
UDI Connect
For tech-driven teams that need to integrate UDI compliance into existing systems.
Offers API-first solutions to automate validation, submissions, and monitoring without disrupting your workflows.
Automate with
UDI Hub
For teams seeking an all-in-one solution to centralize and simplify UDI management.
Automates compliance tasks and provides intuitive tools for data preparation, submission, and tracking.

Industry Leaders Who Trust p36 Solutions
Unlock Cost Savings and Compliance Assurance with our UDI Solutions

Automate Your UDI Workflows
From data validation to submissions, our UDI Solutions automate the most time-consuming tasks.

Reduce UDI Submission Time
Streamline your process with one-click, built-in validations, and error-free data exchange globally.

Expand to Markets
For global regulatory databases like GUDID or EUDAMED, our UDI Solutions simplify compliance.
Industry Leaders' Insights
Trusted by forward-thinking companies to streamline UDI compliance and drive efficiency.
"It’s a great solution, we already recommended p36 to other companies at yearly Regulatory Affairs Events."
"Easy to work with, good communication, great employees."
"Best of breed regarding UDI Database."
Empower Your Compliance Journey with Our Trust Center and Resources
Trust Center
Easily access all essential legal documents and certifications. Everything you need is in one place, ready when you need it.
Regulatory Insights
Stay ahead of change. Get the latest insights on regulatory evolvements affecting your compliance strategies.
Customer Story
Read here how p36’s Expertise is transforming Global UDI Compliance.
